Given this equation (linked in screenshot), which of the following is true if 4.53 moles of C6H14 completely reacts with excess oxygen?
A) 0.755 moles CO2 and 0.162 moles H2O will be formed.
B) 27.1 moles CO2 and 31.7 moles H2O will be formed.
C) 12 moles CO2 and 14 moles H2O will be formed.
D) 54.4 moles CO2 and 63.4 moles H2O will be formed.
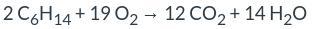
Answers
The correct answer is option D: 54.4 moles CO₂ and 63.4 moles H₂O will be formed when 4.53 moles of C₆H₁₄ completely reacts with excess oxygen.
What is a chemical reaction?
A chemical reaction is a process that leads to the transformation of one chemical substance to another chemical. It involves breaking and forming of chemical bonds between atoms to create new molecules or compounds.
According to the balanced equation given, 2 moles of C₆H₁₄ react with 19 moles of O₂ to produce 12 moles of CO₂ and 14 moles of H₂O.
Therefore, for 4.53 moles of C₆H₁₄ , the amount of O₂ required for complete reaction would be:
(19/2) x 4.53 = 42.9 moles of O₂
Since excess oxygen is present, all the C₆H₁₄ will react, and the number of moles of CO₂ and H₂O produced will be:
CO₂ = 12 x (4.53/2) = 27.2 moles
H₂O = 14 x (4.53/2) = 31.7 moles
Therefore, the answer is D) 54.4 moles CO₂ and 63.4 moles H₂O will be formed.
To find out more about chemical reactions, visit:
https://brainly.com/question/29762834
#SPJ1
Related Questions
A 0.682-gram sample of an unknown weak monoprotic organic acid, HA, was dissolved in sufficient water to make 50.0 mL of solution and was titrated with a 0.135 M NaOH solution. After the addition of 10.6 mL of base, a pH of 5.65 was recorded. The equivalence point was reached after the addition of 27.4 mL of the 0.135 M NaOH.
a. Calculate the number of moles of acid in the original sample.
b. Calculate the molar mass of the organic acid.
c. Calculate the molarity of the unreacted HA remaining in the solution at pH = 5.65.
Answers
a. The number of moles of acid in the original sample is 0.00369. b. The molar mass of the organic acid is 0.135 M. c. The molarity of the unreacted HA remaining in the solution at pH 5.65 is 0.045 M
Calculation:
a. The equivalence point was reached after the addition of 27.4 mL of the 0.135 M NaOH.a.
Moles of NaOH = M × V = 0.135 M × 27.4 mL = 0.00369 moles
Using the balanced equation, we find that the number of moles of HA is equal to the number of moles of NaOH at the equivalence point. HA + NaOH → NaA + HOH0. 00369 moles of NaOH are needed to react with 0.00369 moles of HA.
b. Molar mass of HA = (mass of HA) / (number of moles of HA) = 0.682 g / 0.00369 moles = 184.7 g/molc. Calculate the molarity of the unreacted HA remaining in the solution at pH = 5.65.The pH of the solution was 5.65 after 10.6 mL of NaOH were added.
c. To calculate the molarity of the remaining HA, we first need to find the pKa of the acid.
pH = pKa + log([A-]/[HA])5.65 = pKa + log([A-]/[HA]). We know that at the equivalence point, [A-] = [HA] / 2.
Therefore,[A-] = 0.00369 moles / 2 = 0.00185 moles[Ligand] = (moles of ligand) / (liters of solution). We need to find [HA] in moles/L, so we need to find [A-] in moles/L. We can use the molarity of the NaOH solution to do this. [NaOH] = 0.135 M
moles of NaOH = [NaOH] × (liters of solution)moles of NaOH = 0.135 M × 0.0106 L.
moles of NaOH = 0.00144 moles
moles of HA at pH = 5.65 = moles of HA initially - moles of NaOH added = 0.00369 moles - 0.00144 moles
= 0.00225 moles[HA] = 0.00225 moles / 0.050 L = 0.045 M
To know more about molarity, refer here:
https://brainly.com/question/17238709#
#SPJ11
a substance has a ph that is lower than the ph of household bleach but higher than the ph of egg whites. based on this information, the substance is
Answers
The substance with a pH that is lower than bleach but higher than egg whites would have a pH between 8 and 12.
What is pH?pH is a measure of the acidity or basicity (alkalinity) of a solution. It is measured on a scale from 0 to 14, with a pH of 7 considered neutral. pH values below 7 indicate acidity, while pH values above 7 indicate basicity.
Household bleach has a pH of around 12-13, while egg whites have a pH of around 7-8. Therefore, the substance with a pH that is lower than bleach but higher than egg whites would have a pH between 8 and 12.
Substances with a pH in this range include baking soda (pH around 9), milk of magnesia (pH around 10), and ammonia solution (pH around 11). However, without further information, it is impossible to determine the exact substance with certainty.
Learn more about pH here: https://brainly.com/question/26424076
#SPJ1
what do the dashed lines represent in the illustration of the transition state of a reaction between brch3 and oh--?
Answers
The dashed lines in the illustration of the transition state of a reaction between BrCH3 and OH- represent partial bond formation and bond breaking.
The transition state is the highest energy point in a chemical reaction, where the reactants are transformed into products. In this transition state, the reactants are in a state of instability and are subject to significant energy changes. The transition state is the state at which the energy of the reactants is highest. When the reactants react and form products, the energy is decreased. The transition state is the point at which the reaction barrier must be crossed for the reaction to proceed.
The dashed lines in the illustration represent the partial bond formation and bond breaking that occur during the transition state. The bond formation is represented by the dashed lines going toward the Br and O atoms, which indicates that these atoms are beginning to share electrons. Meanwhile, the dashed lines going away from the Br-C and C-O atoms indicate the bond breaking that is occurring during the reaction. In general, the dashed lines represent the changing bond lengths and bond energies that are occurring during the transition state. This depiction of the transition state is an important tool for chemists to understand how reactions occur and how they can be optimized. Understanding the mechanism of a reaction can help scientists design more efficient and effective reactions, which can be used to synthesize a wide variety of compounds.
Know more about transition state here:
https://brainly.com/question/30835920
#SPJ11
what is the correct way to write the formula of the compound formed by a hydrogen ion and a sulfate ion? group of answer choices h 2 (so 4 ) 2 hso 4 h 2 so 4 h(so 4 ) 2
Answers
The correct way to write the formula of the compound formed by a hydrogen ion and a sulfate ion is c. h2so4.
A compound is a pure substance composed of two or more different atoms chemically bonded in a fixed proportion. The atoms in a compound can be combined in a range of methods and in various ratios. When atoms of two or more elements chemically combine, they form a compound.
The hydrogen ion or proton has a chemical symbol of H+. Chemical formula of sulfate ion. The chemical formula for sulfate ion is SO42-. Formula of the compound formed by a hydrogen ion and a sulfate ion. The formula of the compound formed by a hydrogen ion and a sulfate ion is h2so4.
Learn more about proton at:
https://brainly.com/question/1264222
#SPJ11
if a chemical is spilled onto the face, wait until you have washed the chemicals off before removing your glasses. group of answer choices true false
Answers
Never use a spatula to empty a bottle with solid chemicals. You will contaminate the chemical if you do this. Pour solid into your straight in place of it.
If you spill a chemical on oneself, what should you do?If this solid substance or a liquid containing it comes in touch with your skin, immediately wash it with soap and water to remove any contamination. After cleaning, seek medical assistance if the irritation continues.
What should you do if you just splattered a chemical on someone's face?The area should be immediately thoroughly flushed with water for at least 15 minutes. Try to prevent cross-contamination if flushing your eyes is not necessary.
To know more about solid chemicals visit:-
brainly.com/question/14966269
#SPJ1
write the chemical equation for the ion pairing of sr2 (aq) and c2o42-(aq) leading to their soluble ion pair.
Answers
The chemical equation for the ion pairing of Sr2+ (aq) and C2O42- (aq) leading to their soluble ion pair is given by the following chemical equation: Sr2+ (aq) + C2O42- (aq) ⇌ SrC2O4 (s).
Here, Sr2+ (aq) is an aqueous solution of strontium ions and C2O42- (aq) is an aqueous solution of oxalate ions. When these two solutions are mixed, they undergo a reaction to form a precipitate of strontium oxalate (SrC2O4) which is a soluble ion pair.
The reaction is reversible because the soluble ion pair can dissociate into its constituent ions under certain conditions. The solubility of the ion pair is determined by the equilibrium constant (Ksp) of the reaction which is given by the following equation: Ksp = [Sr2+][C2O42-] where [Sr2+] and [C2O42-] are the concentrations of strontium ions and oxalate ions in the solution, respectively.
Thus, the chemical equation for the ion pairing of Sr2+ (aq) and C2O42- (aq) leading to their soluble ion pair is: Sr2+ (aq) + C2O42- (aq) ⇌ SrC2O4 (s).
Read more about reaction;
https://brainly.com/question/24795637
#SPJ11
the strongest intermolecular forces are nearly as strong as the forces that hold atoms together in a molecule. true false
Answers
The statement "the strongest intermolecular forces are nearly as strong as the forces that hold atoms together in a molecule" is a false statement. The forces that hold atoms together within a molecule are primarily chemical bonds that are incredibly powerful forces.
Intermolecular forces are the forces of attraction and repulsion between different molecules or particles. In contrast, intramolecular forces refer to the forces that hold atoms together within a molecule.
There are three main types of intermolecular forces:
Van der Waals forcesHydrogen bondsDipole-dipole interactionsThese forces are considerably weaker.
The forces that hold atoms together within a molecule are primarily chemical bonds that are incredibly powerful forces. The forces of chemical bonds involve the sharing or transfer of electrons between atoms. Covalent bonds, ionic bonds, and metallic bonds are examples of chemical bonds that hold atoms together in molecules. These bonds are so strong that they are difficult to break.
To learn more about intermolecular forces refer - https://brainly.com/question/20162721
#SPJ11
partial older osteons can be found between complete newer osteons. these partial osteons are referred to as
Answers
Partial, older osteons are cylindrical structures that are found between newer, more complete osteons. These structures, also known as fragments,
consist of concentric layers of lamellae surrounding a central canal, or Haversian canal.
The lamellae and the Haversian canal are formed during the process of osteon remodeling, which involves the removal of old osteons and their replacement with new ones.
The fragments of old osteons that remain in the matrix between new osteons are referred to as “intermediate,” “intermediate osteons,” or “partial osteons.”
They can be distinguished from the newer, complete osteons by their decreased size and lack of a central Haversian canal.
Partial osteons are important for a number of reasons. They help maintain the structural integrity of the bone, provide additional strength and stability, and increase the bone’s resistance to compressive and tensile stresses.
Partial osteons also act as an area of interface between two different age groups of osteons, allowing them to resist shear forces.
Finally, the presence of partial osteons in the bone matrix may increase the rate of healing after fracture or trauma.
to know more about osteons refer here:
https://brainly.com/question/30327119#
#SPJ11
Calculate the molality of a solution that contain 90. 0g of benzoic acid in 350 ml of water
Answers
The molality of a solution that contain 90. 0g of benzoic acid in 350 ml of water is 2.102 mole / kg.
The molarity of a solution is defined as the number of moles of solute dissolved in one liter of solution. Molarity can be expressed as the ratio of a solvent's moles to a solution's total liters. Both the solute and the solvent are part of the solution in calculating the molarity. It is the ratio of the solute moles to the solvent kilograms.
Molarity = Number of moles of solute Volume of solution in liter.
moles of C6H5COOH = 90.0 g / 122.12g/mole
= 0.736 mole
Now we have to calculate the mass of water.
= (350 ml) (1 g/ml) * 1L/ 1000ml
= 0.350 kg
Molarity = 0.736 mole/ 0.350 kg
= 2.102 mole / kg.
To learn more about Molarity
https://brainly.com/question/30404105
#SPJ4
hydrogen peroxide decomposes into water and oxygen at constant pressure by the following reaction 2 h2o2--> 2 h2o o2 deltah -196 kj. calculate the value of q in kj in this exothermic reaction when 2.00g of hydrogen peroxide decomposes at constant pressure
Answers
The decomposition of hydrogen peroxide into water and oxygen is an exothermic reaction, and its value can be calculated using the enthalpy change. The enthalpy change is the amount of energy released or absorbed during a chemical reaction. In this reaction, the enthalpy change is -196 kJ, which means that the reaction releases 196 kJ of
energy when 2 moles of hydrogen peroxide are decomposed into water and oxygen at constant pressure.
Now, we need to calculate the value of q in kJ when 2.00 g of hydrogen peroxide is decomposed at constant pressure. The molar mass of hydrogen peroxide (H2O2) is 34.0147 g/mol. So, the number of moles of hydrogen peroxide can be
calculated as follows:
Number of moles of H2O2 = mass of H2O2 / molar mass of H2O2
Number of moles of H2O2 = 2.00 g / 34.0147 g/mol
Number of moles of H2O2 = 0.05878 mol
As per the balanced chemical equation, 2 moles of hydrogen peroxide are required to produce 2 moles of water and 1 mole of oxygen. So, the number of moles of water and oxygen produced can be calculated as follows:
Number of moles of H2O = 2 x 0.05878 mol = 0.11756 mol
Number of moles of O2 = 1 x 0.05878 mol = 0.05878 mol
The enthalpy change is -196 kJ per 2 moles of hydrogen peroxide. So, the enthalpy change per 1 mole of hydrogen peroxide can be calculated as follows:
Enthalpy change per 1 mole of H2O2 = -196 kJ / 2 mol
Enthalpy change per 1 mole of H2O2 = -98 kJ/mol
For more similar questions on topic hydrogen peroxide
brainly.com/question/11743179
#SPJ11
give the charge of the stable ion formed by each of the following. include the sign ( or -) and magnitude (numerical value) of the charge in every case. (note: give only the charge, not the formula of the ion.) 1. a group 2a metal 2. a group 3a metal
Answers
A group 2A metal will form a stable ion with a charge of +2. Examples of group 2A metals include magnesium (Mg), calcium (Ca), and strontium (Sr).
A group 3A metal will form a stable ion with a charge of +3. Examples of group 3A metals include boron (B), aluminum (Al), and gallium (Ga).
For more questions like charge visit the link below:
1. A metal of group 2A, plus
2. A metal from group 3A, - 3+
A What is charge?
Both positive and negative charges are possible. We are aware that a positive charge is created when a species has more protons than electrons. A negative ion, on the other hand, is one that has more electrons than protons.
We now understand that metals mostly produce positive ions. The group that the metal belongs to in the periodic table determines how much charge is on the ions.
The ions' charges are as follows:
1. A metal of group 2A, plus
2. A metal from group 3A, - 3+
https://brainly.com/question/28531714
#SPJ11
The chemical weathering process known as oxidation would be most effective in the breakdown of which of the following Earth minerals?
pyroxenes
quartz
calcite
halite
feldspar
Answers
The chemical weathering process known as oxidation would be most effective in the breakdown of pyroxenes, quartz, calcite, and feldspar Earth minerals.
Oxidation is a chemical reaction between oxygen and other substances in the environment which causes a breakdown of the Earth minerals, resulting in their decomposition. Halite, or sodium chloride, is an example of a mineral that is not affected by the oxidation process.
Learn more about the chemical weathering process at brainly.com/question/9621344
#SPJ11
Iron oxide, known as hematite, is the main source of iron for the steel industry. It is created when iron and oxygen combine in a thermal decomposed reaction. It can be described using the equation 4Fe + 3O2 = 2Fe2O3. If 19.2 g of O2 reacts with iron to form 63.84 g of oxide, how much iron in grams was used in the reaction?
Answers
The balanced equation for the reaction is:
4Fe + 3O2 -> 2Fe2O3
We are given the mass of oxygen and the mass of iron oxide produced. To find the mass of iron used in the reaction, we need to use stoichiometry to relate the masses of the reactants and products.
First, we can calculate the molar mass of Fe2O3:
Fe2O3 = 2(55.845 g/mol) + 3(16.00 g/mol) = 159.69 g/mol
Next, we can use the mass of iron oxide produced to find the number of moles of Fe2O3:
63.84 g Fe2O3 × (1 mol Fe2O3/159.69 g Fe2O3) = 0.400 mol Fe2O3
Since the reaction produces 2 moles of Fe2O3 for every 4 moles of Fe, we can find the number of moles of Fe:
0.400 mol Fe2O3 × (4 mol Fe / 2 mol Fe2O3) = 0.800 mol Fe
Finally, we can use the molar mass of Fe to convert the number of moles to grams:
0.800 mol Fe × 55.845 g/mol = 44.68 g Fe
Therefore, 44.68 grams of iron were used in the reaction.
the density of acetic anhydride (c4h6o3) is 1.08 g/ml. if 5.65 ml of acetic anhydride is used in the experiment, then how many moles of acetic anhydride was used?
Answers
The number of moles of acetic anhydride used is 0.06 moles.
The number of moles of acetic anhydride (C₄H₆O₃) can be calculated by multiplying the given volume by the given density, and then dividing the result by the molar mass of acetic anhydride. The molar mass of acetic anhydride (C₄H₆O₃) is the sum of the atomic weights of each element.
In this case, we have : Volume (V) = 5.65 mL, Density (ρ) = 1.08 g/mL, and Molar mass (M) = 102.09 g/mol
Solving for the number of moles, we get:
Number of moles (n) = V x ρ / M
n = 5.65 mL x 1.08 g/mL / 102.09 g/mol
n = 0.06 moles of acetic anhydride
Learn more about density here: https://brainly.com/question/1354972.
#SPJ11
How much KNO3 will dissolve in 200 grams H2O at 70 C
Answers
The red line shows that at 70 °C, 200 g of water will be saturated with about 140 g or potassium nitrate.
How does solubility in 100 grammes of water become calculated?This mass of a compound would be divided by mass of the solvent, and then divided by 100 g to determine its solubility. This calculation will give the solubility of the substance in g/100g.
How does the temperature affect KNO3's solubility in water?The curves demonstrate that when temperature rises, solubility of any and all three solutes increases. The most noticeable increase in solubility is for potassium nitrate, which goes from about 30 g per 100 g of water from over 200 grams per 100 grams of water.
To know more about potassium visit:
https://brainly.com/question/13321031
#SPJ1
Use the electron-transfer method to balance this redox equation: Aluminum metal reacts with hydrochloric acid to produce aluminum III chloride and hydrogen gas.
Answers
The balanced equation for the redox reaction when Al metal reacts with HCl is 2Al + 6HCl -> 2[tex]AlCl_{3}[/tex] + 3[tex]H_{2}[/tex].
How to balance redox reactions via Electron Transfer Method?To balance the redox equation for the reaction between aluminum metal and hydrochloric acid using the electron transfer method, follow these steps:
Step 1: Write the unbalanced equation:
Al + HCl -> [tex]AlCl_{3}[/tex] + [tex]H_{2}[/tex]
Step 2: Separate the equation into half-reactions:
Oxidation half-reaction: Al -> [tex]Al^{3+}[/tex]
Reduction half-reaction: [tex]H^{+}[/tex] -> [tex]H_{2}[/tex]
Step 3: Balance the atoms in each half-reaction, except for oxygen and hydrogen:
Oxidation half-reaction: Al -> [tex]Al^{3+}[/tex] (already balanced)
Reduction half-reaction: 2[tex]H^{+}[/tex] -> [tex]H_{2}[/tex]
Step 4: Balance the charges in each half-reaction by adding electrons:
Oxidation half-reaction: Al -> [tex]Al^{3+}[/tex] + 3[tex]e^{-}[/tex]
Reduction half-reaction: 2[tex]H^{+}[/tex] + 2[tex]e^{-}[/tex] -> [tex]H_{2}[/tex]
Step 5: Equalize the number of electrons transferred in both half-reactions by multiplying the half-reactions by appropriate factors:
Oxidation half-reaction: 2(Al -> [tex]Al^{3+}[/tex] + 3[tex]e^{-}[/tex] ) -> 2Al -> 2[tex]Al^{3+}[/tex] + 6[tex]e^{-}[/tex]
Reduction half-reaction: 3(2[tex]H^{+}[/tex] + 2[tex]e^{-}[/tex] -> H2) -> 6[tex]H^{+}[/tex] + 6[tex]e^{-}[/tex] -> [tex]H_{2}[/tex]
Step 6: Add the balanced half-reactions back together:
2Al + 6[tex]H^{+}[/tex] -> 2[tex]Al^{3+}[/tex] + 3[tex]H_{2}[/tex]
Step 7: Add back the spectator ions (chloride ions) to complete the balanced equation:
2Al + 6HCl -> 2[tex]AlCl_{3}[/tex] + 3[tex]H_{2}[/tex]
The balanced redox equation using the electron transfer method is:
2Al + 6HCl -> 2[tex]AlCl_{3}[/tex] + 3[tex]H_{2}[/tex]
To know more about Electron Transfer Method:
https://brainly.com/question/30897672
#SPJ11
which of the following processes is not spontaneous at room temperature? ice melting salt dissolving in water hot coffee cooling down hot tea getting hotter silver tarnishing
Answers
At room temperature, ice melting, salt dissolving in water, hot coffee cooling down, and hot tea getting hotter are all spontaneous processes. Silver tarnishing, however, is not a spontaneous process at room temperature.
What is spontaneity?Spontaneity is defined as a procedure that happens without external impact. The procedures that occur without any interference are known as spontaneous procedures, and the ones that occur only with external influence are called non-spontaneous procedures. The distinction between spontaneous and non-spontaneous processes is the focus of thermodynamics. Processes that happen on their own are referred to as spontaneous.
Examples of spontaneous processes are Ice melting, Salt dissolving in water, and hot tea getting hotter.
An example of a non-spontaneous process is Silver tarnishing.
The conditions of the spontaneous processes are ΔS > 0ΔH < 0ΔG < 0 at room temperature.
to know more about the Spontaneous process:
https://brainly.com/question/1109991
#SPJ11
a solution is prepared by dissolving 99.7 g of nai in enough water to form 895 ml of solution. calculate the mass % of the solution if the density of the solution is 1.06 g/ml.
Answers
The mass % of the solution if the density of the solution is 1.06 g/ml is 10.51%
The mass of NaI = 99.7 g
Volume of the solution = 895 ml
Density of the solution = 1.06 g/ml
To calculate the mass % of the solution, we have to calculate the mass of the solution first.
Step-by-step explanation:
The formula for density is given by:
Density = Mass/Volume
Or,
Mass = Density × Volume
Now, we will calculate the mass of the solution.
Mass = Density × Volume
= 1.06 × 895= 948.7 g
Now, we will calculate the mass % of the solution.
Mass % = (Mass of solute/Total mass of solution) × 100
Mass of solute = 99.7 g
Total mass of solution = 948.7 g
Mass % = (99.7/948.7) × 100
= 10.51%
Therefore, the mass % of the solution is 10.51%.
Learn more about mass %: https://brainly.com/question/26150306
#SPJ11
how many unique sets of 4 quantum numbers are there to represent the electrons in the 4f subshell? remember that the pauli exclusion principle states that each electron must have its own unique set of 4 quantum numbers.
Answers
The number of unique sets of 4 quantum numbers to represent the electrons in the 4f subshell is 70.
The four quantum numbers that make up an electron's set are the:
(i) principal quantum number (n)
(ii) angular momentum quantum number (l)
(iii) magnetic quantum number (m_l)
(iv) spin quantum number (m_s).
Each of these electrons has a limited range of the above numbers in their respective shell.
The principal quantum number for all the electrons in the 4f subshell is 4.
The angular momentum quantum number has a value of 3 corresponding to the f subshell.
The magnetic quantum number has a range of -3 through +3 for the electrons in the f subshell.
The spin quantum number has a range of -1/2 or +1/2.
Even if the principal quantum number and angular momentum quantum number are the same for all the electrons, the other two factors contribute to each electron having a unique set of quantum numbers.
Therefore, when these four quantum numbers are combined, they make up 70 unique sets of 4 quantum numbers that can be used to represent the electrons in the 4f subshell, in accordance with the Pauli Exclusion Principle.
To know more about quantum numbers, refer here:
https://brainly.com/question/16977590#
#SPJ11
true or false: when two solutions containing ions as solutes are combined and a reaction occurs, it is always a single-replacement reaction.
Answers
The statement that "when two solutions containing ions as solutes are combined and a reaction occurs, it is always a single-replacement reaction" is False.
When two solutions containing ions as solutes are combined and a reaction occurs, it is not always a single-replacement reaction.
The type of reaction that will occur depends on the reactants and the conditions of the reaction.
For example, if two solutions containing different metal ions are mixed together, a double-replacement reaction may occur, in which two ionic compounds are formed.
Similarly, a precipitation reaction may occur if the combination of the two solutions produces an insoluble product.
In general, single-replacement reactions involve one element replacing another element in a compound, and occur when one of the reactants is an elemental solid, such as a metal.
To know more about solutes, refer here:
https://brainly.com/question/7932885#
#SPJ11
43.5-grams of barium sulfate is formed from the reaction of barium nitrate and sodium sulfate. how many moles of sodium sulfate reacted?
Answers
Answer: 0.25 moles
Explanation:
To determine the number of moles of sodium sulfate that reacted, we need to first identify the limiting reagent in the reaction between barium nitrate and sodium sulfate. The balanced chemical equation for the reaction is:
Ba(NO3)2 + Na2SO4 → BaSO4 + 2NaNO3
The stoichiometry of the reaction tells us that one mole of barium sulfate is produced for every mole of sodium sulfate that reacts. Therefore, we can calculate the number of moles of sodium sulfate that reacted by dividing the mass of barium sulfate produced by its molar mass:
43.5 g BaSO4 × (1 mol BaSO4/233.4 g BaSO4) = 0.186 mol BaSO4
Since one mole of sodium sulfate reacts with one mole of barium sulfate, the number of moles of sodium sulfate that reacted is also 0.186 mol.
However, this is the number of moles of barium sulfate that was formed. To determine the number of moles of sodium sulfate that reacted, we need to use the stoichiometry of the balanced chemical equation. The ratio of the coefficients of sodium sulfate to barium sulfate in the balanced equation is 1:1. Therefore, the number of moles of sodium sulfate that reacted is also 0.186 mol.
The term mole concept is used here to determine the moles of sodium sulfate reacted. The moles of sodium sulfate formed from 43.5-grams of barium sulfate is 0.186 moles.
What is a mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
Here the balanced equation is:
Ba(NO₃)₂ + Na₂SO₄ → BaSO₄ + 2NaNO₃
The stoichiometry of the reaction tells us that one mole of barium sulfate is produced for every mole of sodium sulfate that reacts. Therefore, we can calculate the number of moles of sodium sulfate that reacted by dividing the mass of barium sulfate produced by its molar mass:
43.5 g BaSO4 × (1 mol BaSO4/233.4 g BaSO4) = 0.186 mol BaSO4
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ2
how can you tell by looking at a graph which reaction (forward or reverse) is favored (i.e. faster when the concentrations of reactants and products are equal)?
Answers
The forward reaction is favored when the graph shows that the reactant concentration is higher than the product concentration.
To determine which reaction is favored, examine the graph and look at the concentrations of reactants and products at equilibrium. If the reactant concentration is higher, the forward reaction is favored. Conversely, if the product concentration is higher, the reverse reaction is favored.
A graph can help you visualize the reactants and products of a reaction at equilibrium. The y-axis of the graph typically indicates the concentration of the reactants or products, and the x-axis of the graph indicates the reaction rate.
At equilibrium, the reaction rate is 0, meaning that the reactants and products are neither increasing nor decreasing in concentration. By looking at the concentrations of the reactants and products at equilibrium on the graph, you can determine which reaction is favored.
If the reactant concentration is higher than the product concentration, then the forward reaction is favored. This means that the forward reaction occurs more quickly than the reverse reaction when the concentrations of the reactants and products are equal.
Conversely, if the product concentration is higher than the reactant concentration, then the reverse reaction is favored.
To know more about forward reaction click on below link:
https://brainly.com/question/8592296#
#SPJ11
to what volume (in ml) would you need to dilute 45.0 ml of a 1.20 m solution of nabr to make a 0.0400 m solution of nabr?
Answers
To dilute 45.0 ml of a 1.20 M solution of NaBr to a 0.0400 M solution, you need to add enough water to a total volume of 226.25 ml.
The dilution formula is M1V1 = M2V2, where M1 and V1 are the initial molarity and volume of the solution and M2 and V2 are the desired molarity and volume of the dilute solution.
Calculate V2 (the desired volume) by rearranging the equation and solving for V2: V2 = (M1V1) / M2.
V2 = (1.20M * 45.0ml) / 0.0400M = 226.25ml.
Therefore, to create a 0.0400 M solution of NaBr from a 1.20 M solution of NaBr, you need to add enough water to a total volume of 226.25 ml.
to know more about solution refer here:
https://brainly.com/question/30665317#
#SPJ11
how many milliliters of 11.5 m hcl(aq) 11.5 m hcl ( aq ) are needed to prepare 855.0 ml 855.0 ml of 1.00 m hcl(aq)?
Answers
74.3 mililiters of 11.5 M HCl (aq) is required to prepare 855.0 mL of 1.00 M HCl (aq).
Dilution formulaTo calculate how many milliliters of 11.5 M HCl (aq) are required to prepare 855.0 mL of 1.00 M HCl (aq), we will utilize the dilution formula.
The formula for dilution is:
C₁V₁ = C₂V₂
Where:
C₁ = initial concentration
V₁ = initial volume
C₂ = final concentration
V₂ = final volumeIn this case
C₁ = 11.5 M
V₁ = ?
C₂ = 1.00 M
V₂ = 855.0 mL
Firstly, let's rearrange the formula and solve for V₁ by substituting the given values. We will then calculate the value of V₁:
C₁V₁ = C₂V₂
11.5 M V₁ = 1.00 M × 0.855 L
V₁ = 1.00 M × 0.855 L / 11.5 M = 0.07434 l or 74.34 ml
learn more about dilution
https://brainly.com/question/27097060
#SPJ11
on the basis of the information in the chart and what you know about atomic structure, which elements form stable but reactive diatomic gases?
Answers
On the basis of the information in the chart and what you know about atomic structure, the elements that form stable but reactive diatomic gases are hydrogen, nitrogen, oxygen, and fluorine.
A diatomic element is an element that can form two-atom molecules. The diatomic elements' covalent bonds keep these molecules together. The prefix "di-" in "diatomic" indicates two and diatomic gases, or simply diatomics, are gases consisting of molecules with two atoms of the same or different chemical elements in their molecule.
The four most well-known diatomic elements are hydrogen (H2), nitrogen (N2), oxygen (O2), and fluorine (F2). The general formula for diatomic molecules is X2, where X represents an element. Some other examples include chlorine (Cl2), bromine (Br2), and iodine (I2). A stable but reactive diatomic gas is a diatomic gas that is chemically stable enough to exist as a molecule but is chemically reactive. These diatomic gases usually do not react spontaneously or violently, but they may react with other chemicals under the proper conditions.
Learn more about covalent bonds at:
https://brainly.com/question/11674395
#SPJ11
determine the mass percent (to the hundredths place) of h in sodium bicarbonate (nahco3). 14.30 27.36 1.20 57.14 19.05
Answers
The mass percent of hydrogen in sodium bicarbonate (NaHCO3) is 1.20% (to the hundredths place).
To determine the mass percent of hydrogen (H) in sodium bicarbonate (NaHCO3), we need to first calculate the molar mass of NaHCO3, which is:
NaHCO3 = 1(Na) + 1(H) + 1(C) + 3(O)
= 23.00 + 1.01 + 12.01 + (3 x 16.00)
= 84.01 g/mol
The mass of hydrogen in one mole of NaHCO3 is 1.01 g, since there is only one hydrogen atom in each molecule of NaHCO3.
Therefore, the mass percent of hydrogen in NaHCO3 can be calculated as follows:
mass percent H = (mass of H / mass of NaHCO3) x 100%
= (1.01 g / 84.01 g) x 100%
= 1.20%
For more question on sodium bicarbonate click on
https://brainly.com/question/1596599
#SPJ11
147 grams of argon to liters
Answers
Answer:
Explanation:
3.6797837188344116 mol
roup 13 nitrides are isostructural and exhibit the layered graphite structure group of answer choices true false
Answers
The given statement, Group 13 nitrides are isostructural and exhibit the layered graphite structure is true because Group 13 nitrides have a hexagonal close-packed layer of nitrogen atoms, with alternating layers of boron, aluminum, or gallium atoms, resulting in a layered structure that resembles graphite due to comparable atomic radii and electronegativities of the elements.
Group 13 nitrides, such as boron nitride (BN), aluminum nitride (AlN), and gallium nitride (GaN), are isostructural and exhibit the layered graphite structure. The basic building block of the crystal structure is a hexagonal close-packed layer of nitrogen atoms, with alternating layers of boron, aluminum, or gallium atoms. This results in a layered structure that resembles that of graphite. The similarities between the crystal structures of these materials can be attributed to the comparable atomic radii and electronegativities of the Group 13 elements.
To know more about nitrides, here
brainly.com/question/16501916
#SPJ4
--The complete question is, Group 13 nitrides are isostructural and exhibit the layered graphite structure group of answer choices true false.--
how many different alkenes result when 2-bromohexane is treated with a strong base? select answer from the options below 1 2 3 4
Answers
When 2-bromohexane is treated with a strong base the alkenes that would result is given as 1
What alkenes would resultWhen 2-bromohexane is treated with a strong base, such as sodium ethoxide (NaOEt) or sodium hydroxide (NaOH), it undergoes elimination reaction (also called dehydrohalogenation) to form different alkenes.
The product(s) of the reaction depend on the position of the β-carbon (the carbon next to the bromine atom) that undergoes deprotonation. Since there are two β-carbons in 2-bromohexane, two different alkenes can be formed.
Read more on alkenes here:https://brainly.com/question/29120960
#SPJ1
mariela finds that an element's most stable ion forms x2 . the ion of element x has a mass number of 137 and 54 electrons. a) (2 pts) what is the identity of the element x? b) (2 pts) how many neutrons does it have?
Answers
Answer: a) The identity of element X is Xenon. b) There are 83 neutrons in element X.
In order to identify the element X, let's first find its atomic number. The number of electrons in the neutral atom is equivalent to the atomic number of the particular element. The ion of element X has a total of 54 electrons, so X has 54 protons, implying that X's atomic number is 54. The atomic number of a chemical element is the number of protons in its atomic nucleus.
b) The number of neutrons in an atom is equal to the mass number minus the atomic number of the element. Thus, the number of neutrons in element X can be determined by subtracting 54 from 137, which gives 83 neutrons inside atom of elements.
Know more about Xenon here:
https://brainly.com/question/5516586
#SPJ11